
Von Maxim Sharakin
This year, I was selected as a candidate from the Winterthur Institute of Health Economics to participate in the Swissnex staff mobility program to learn from researchers in Japan about their experiences working in the field of Health Technology Assessment and Cost-effectiveness analysis (CEA). One of the topics that was raised during the discussions was Japan’s pioneering role in the use of CEA for the pricing of medicines, implemented in 2019 with the objective of containing health care expenditures. Such a system could potentially be transferrable to the Swiss setting. I would like to introduce you to the pharmaceutical pricing system in Japan.
New medicines
In Japan, once a medication is approved based on efficacy and safety data, its reimbursement by the national health insurance starts within 60-90 days. In Switzerland the number corresponds to a maximum of 60 days, however, it often can reach a length of 200 days.[1]
Before reimbursement starts, the price of a new medication is established. The price depends on whether there exist comparable medications in the market (based on efficacy, pharmacological action, etc.):
- If a comparable medication exists, the price of the new medication is set to the price of the already available medication.
- In some cases, a corrective premium is added to the matched price because the medication adds value in terms of:
- Innovativeness: high efficacy or safety, or new mechanism of action (premium rate 70-120%).
- Usefulness: more convenient administration method (5-60%).
- Marketability: targets a rare disease (5-20%).
- Child premium: dosage and administration targeted at children (5-20%).
- Early premium: approved in Japan before other countries (10-20%).
- If a comparable medication does not exist, the price is calculated based on research and development costs, distribution costs and the profit margin, by taking an average of the pharmaceutical manufacturing industry costs for the most recent three years, plus the manufacturing costs.
- Based on the same factors described for the case when a comparable drug exists in the market, a corrective premium may be added to the price of the new drug that doesn’t have a comparator. The amount of the premium is calculated as: price before the premium multiplied by the premium rate multiplied by the premium coefficient. The premium coefficient equals 1.0, when at least 80% of the components of the price of the medication before the premium is disclosed, 0.6 when this number reaches 50-80%, 0.2 for less than 50%.
Price revision
After the price of a medicine has been established, it is revised every two years. In Switzerland, prices are revised every three years and after the patent expiration. Suppliers typically can increase the price of the medicines to make up their profit. Thus, a survey of a majority of suppliers is conducted to collect information on the average price at which they sell to healthcare institutions (without consumption tax), which then is multiplied by 1 + consumption tax and 2% of the medicine price is added.
Expensive medications
A complimentary price-adjustment method was introduced in Japan in 2019 for medicines with the highest financial impact. This method is called CEA, and it helps to evaluate the value of a medicine for society based on its efficacy, safety and cost profile. Only the premium part of the price can be adjusted. That is, if for example a medicine is deemed extremely useful, a premium of 20% would be added to the price of the medicine. Then, only the 20% of the price would be targeted.
Medicines are considered for CEA when
- Products have annual peak quarterly sales of ≥ Japanese yen (JPY) 10 billion (CHF 70 million approx.)
- Products have annual peak quarterly sales of JPY 5 billion (CHF 35 million approx.) to JPY 10 billion (CHF 70 million approx.), selected as candidates and evaluated based on available human resource capacity.
- Products have substantially high prices or when new efficacy data becomes available.
- Products have annual actual sales of more than JPY 100 billion (CHF 700 million approx.)
Note 1: Excluded are medicines for children and treatment of rare diseases.
Note 2: If there exists a comparable medicine to that included for CEA, all comparable medicines will also be included into the CEA.
The main measure of benefit used in a CEA is the quality-adjusted life year (QALY), which allows to measure the additional quality of life and life years attributed to a medicine. In Switzerland, CEA is not used for the pricing of medicines, but it can be used for reimbursement decisions. However, there is no formal cost-effectiveness threshold that allows to decide about the reimbursement of a medicine. In Japan, the threshold of JPY 5 million per QALY (CHF 35’000) is used for pricing decisions. Beyond this threshold, the price of the medicines will have to be adjusted (see image at the bottom).
As an example, if a medicine that costs JPY 120 with a 20 percent premium (JPY 24) is calculated to have a cost of JPY 7.6 million per QALY, the adjustment rate for the premium will be 0.4 (see image at the bottom). Then 120 – 24*(1 – 0.4), as per the formula below would render a new adjusted price of JPY 105.6. An important limitation is that the entire price of the drug can never be reduced by more than 15%.
Price with adjusted premium = Old price with premium – Premium * (1 – adjustment rate)
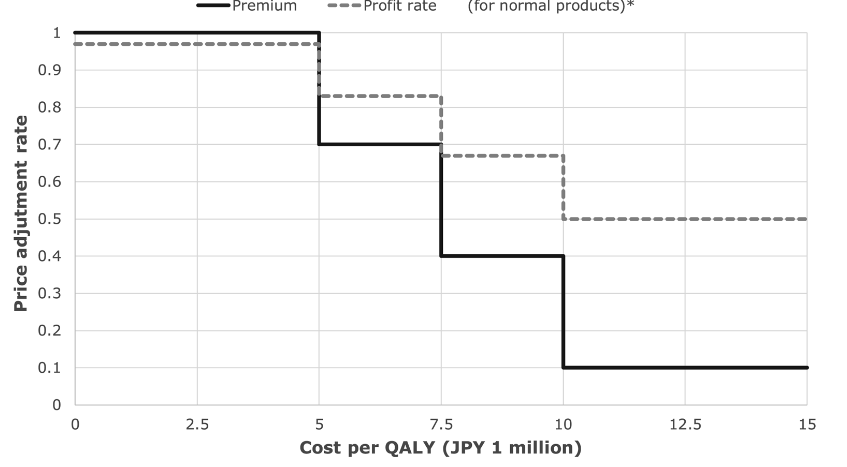
Source: Shiroiwa 2020
2Maxim Sharakin is Scientific Associate in Health Technology Assessment (HTA) and Health-Economic Assessments.
References:
[1] Interpharma. The authorisation and reimbursement process. Retrieved from: https://www.interpharma.ch/themen/der-patient-im-mittelpunkt/patientenzugang/bag-verguetungsprozess/?lang=en
[2] Shiroiwa, T. (2020). Cost-effectiveness evaluation for pricing medicines and devices: A new value-based price adjustment system in Japan. International Journal of Technology Assessment in Health Care, 36(3), 270-276. doi:10.1017/S0266462320000264