Earlier on this blog, I introduced the objectives and planned methodology for our project on soot-vapor interactions in jet engine exhaust, which forms a core part of my Ph.D. work at METENIA and ETH. Since then, I’ve had the opportunity to present some of our first experimental findings at EGU25 (European Geosciences Union General Assembly 2025). I’m excited to now share a summary of what we’ve learned so far.
Why study soot – vapor interactions?
Jet engines release a complex mixture of gases and particles into the atmosphere, including CO₂, NOₓ, VOCs (volatile organic compounds), water vapor, and soot. Although soot particles contribute only a small fraction of total mass emissions, their impacts are significant, from influencing air quality to promoting contrail formation and affecting the Earth’s radiation balance.
One aspect of soot behavior which is often overlooked is its ability to act as a carrier for atmospheric vapors. As jet exhaust cools, vapors such as VOCs and water vapor can adsorb onto soot particle surfaces, altering their physicochemical properties and atmospheric lifetime. Understanding these adsorption and desorption processes is critical for accurate models of soot aging and transport.
From planned methodology to first results
In my earlier post, I described the sample generation and characterization methodologies, including:
- High-throughput generation of aircraft-like soot at ETH.
- High-temperature oxidation treatments for model soot, using temperature and oxygen concentrations similar to those in engine plumes. These treatments allow us to modify the soot precursor’s core-shell structure, affecting its porosity and surface area.
- A set of analytical tools to study soot morphology (SEM: scanning electron microscope), structure (Raman spectroscopy and XRD: X-ray diffraction), and surface chemistry (TG-MS: thermogravimetric-mass spectrometry and XPS: X-ray photoelectron spectroscopy).
This study focus specifically on bulk soot properties, with the hope that our findings can be linked to aerosol behavior, as the observed relationships can be extended down to very low pollutant levels, which is typical in real-world exhaust. Our goal is to identify fundamental relationships between soot structure, surface chemistry, and the interactions with pollutants (VOCs and water), providing insights into soot aging in engine exhaust plumes.
With this detailed characterization data, we have now begun investigating how these soot particles interact with vapors under controlled conditions, starting with single-component adsorption experiments.
Key findings presented at EGU25
We focused on two representative vapor types:
- aromatic VOCs (benzene, toluene), important constituents of jet exhaust,
- water vapor, ubiquitous in the plume and critical for contrail processes.
Here are some highlights from our preliminary results:
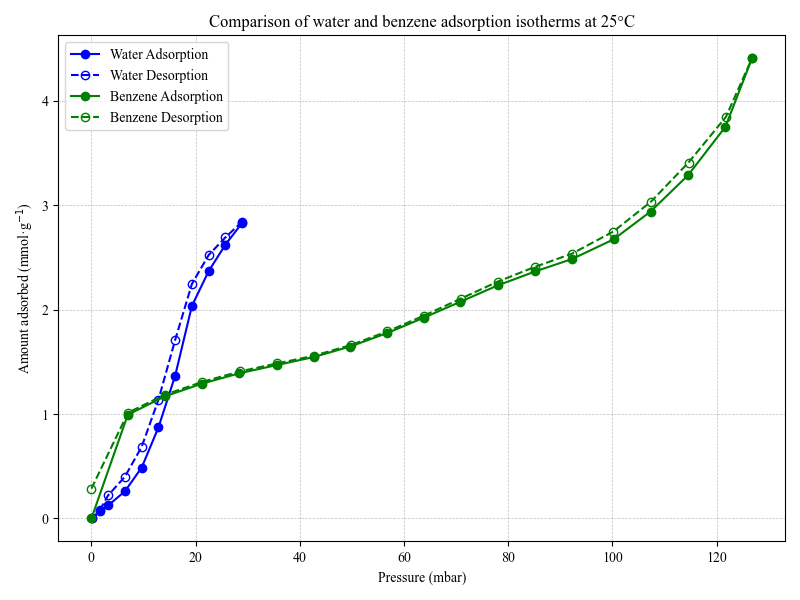
- We found that soot adsorbs VOCs following a pattern typical for surfaces with many available adsorption sites (knowns as a “Type II isotherm”). This behavior is largely due to the chemical groups on the soot surface and its large surface area. Together, these factors confirm that soot particles can take up substantial amounts of VOCs even at low vapor pressures. Thermodynamic analysis showed that the adsorption enthalpies ranged from 31.8–45.4 kJ/mol at low surface coverage. These moderate energy values suggest that the molecules are held on the surface mainly through physical interactions, rather than forming strong chemical bonds.
- Beyond thermodynamics, the adsorption kinetics also revealed interesting behavior. The VOC uptake followed a linear driving force (LDF) model with fast adsorption rates (average rate constant k ≈ 0.016 s⁻¹), suggesting rapid filling of interparticle voids.
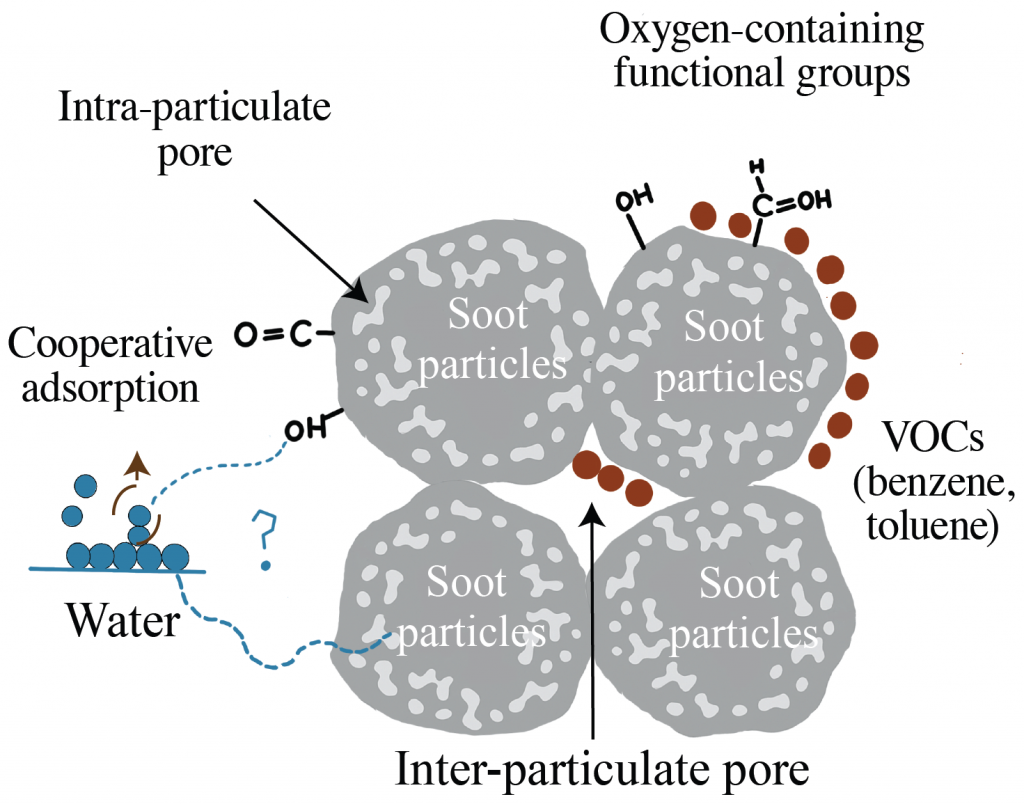
- When looking at water vapor, the isotherm showed a Type V shape, which is typically associated with weak initial adsorption followed by stronger uptake at higher pressures, indicating possible cooperative adsorption. This may reflect diffusion into confined spaces like internal pores, or adsorption onto surface groups and within interparticle voids, where the binding strength varies.
- In terms of rate, water vapor adsorption was noticeably slower (average k ≈ 0.001 s⁻¹) and followed a stretched exponential trend. This type of behaviour suggests that the adsorption process doesn’t happen at one uniform rate, but rather involves a range of rates, likely because water molecules interact with different types of sites or move through regions with varying accessibility.
Why it matters
These results show how soot’s surface chemistry and pore structure govern its interactions with atmospheric vapors, indicating the way soot ages and influences the environment after emission.
Importantly, we now have direct evidence that:
- Jet soot can serve as an efficient temporary sink for VOCs in the exhaust plume due to rapid adsorption.
- The kinetics and extent of water vapor uptake are strongly linked to the internal structure of the particles, and are generally 16 times slower than benzene.
What’s next?
We are now moving toward:
- Carrying out controlled oxidation treatments to gradually change the structure and surface chemistry of model soot samples. By studying the adsorption and desorption processes of VOCs and water, we hope to better understand how these changes influence their interactions with soot.
- Exploring how soot changes during desorption, using temperature-dependent XRD and XPS to observe how its crystalline structure and surface chemistry change.
Stay tuned for more updates as this project progresses, and thanks to everyone who visited our poster and shared valuable feedback at EGU25!